Submitted by Ascension Lourdes and UHS
BINGHAMTON — With the U.S. Food and Drug Administration (FDA) having issued emergency-use authorization (EUA) for two COVID-19 vaccines, Lourdes and United Health Services (UHS) are implementing a comprehensive vaccine-distribution plan that is consistent with federal, state, and local guidance.
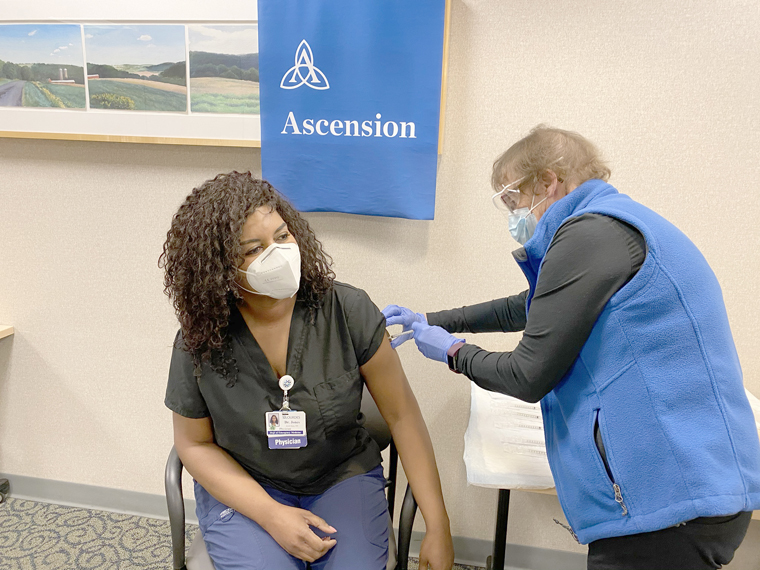
The first recipient for Lourdes was Dr. Jewel Jones, an ER doctor, and for UHS, Dr. Zach Jones, Infectious Disease.
“I was very excited to get the vaccine because I believe that change starts with me,” said Dr. Jewel Jones. “I believe in being an example for my organization and the community. And I feel it’s important I stay healthy so I can be there for those we serve at Lourdes. I’m encouraging my colleagues to get the vaccination, and once it’s available, for the public as well. I’m thankful for my fellow caregivers and their selflessness in the dedicated patient care they deliver every day.”
“I would strongly encourage anyone who qualifies to get vaccinated to get vaccinated,” said Dr. Zach Jones. “Studies for both vaccines indicate they are safe and highly effective. The Pfizer and Moderna vaccines are both approved for anyone over the age of 16 who is not pregnant or breast-feeding.”
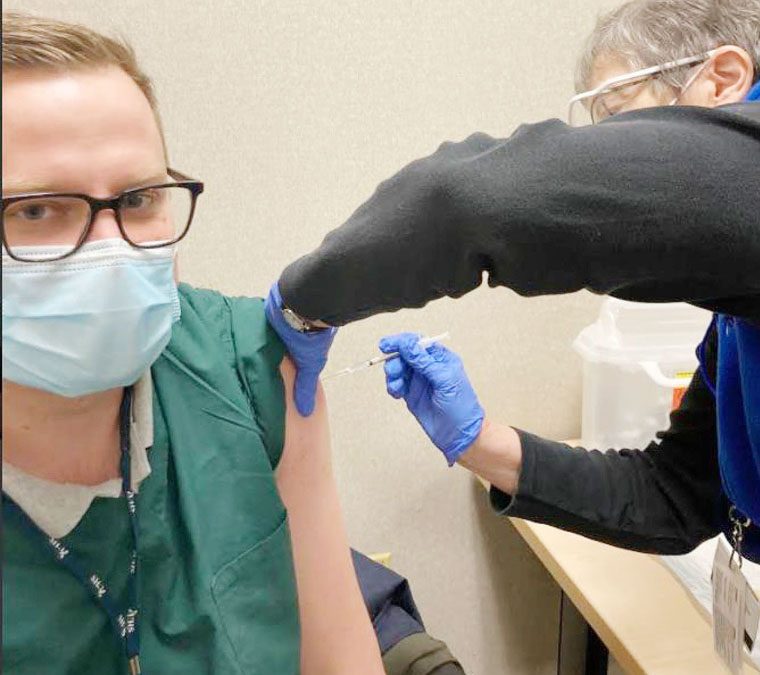
Lourdes ER Dr. Jewel Jones receives the COVID-19 vaccine. Innoculated (Submitted photo)
In accordance with these guidelines, among the first group eligible to receive the vaccines are front-line caregivers — particularly those serving in emergency departments, COVID-19 units, and intensive-care units. Lourdes and UHS anticipate the remainder of its staff will be eligible for the vaccine as more doses become available and the distribution process progresses.
Vaccines from Pfizer and Moderna have demonstrated safety and effectiveness, and the U.S. Food and Drug Administration (FDA) has authorized both vaccines for emergency use.
“We strongly encourage all our employees to receive a COVID-19 vaccine when it is made available to them,” said John Carrigg, president and CEO, United Health Services. “This is the right thing to do to protect our healthcare workers and those we care for in our community. We are thankful for all who have made this vaccine possible and for the continued dedication of our selfless caregivers.”
All approved vaccines require extensive research, documentation, and closely monitored clinical trials to determine effectiveness and safety before being submitted by pharmaceutical companies for approval. Both UHS Medical Staff and Ascension have been involved in some of these clinical trials.
“It’s vitally important that people continue to access the healthcare they need, for both emergencies and chronic conditions,” said Kathy Connerton, president and CEO, Ascension Lourdes. “By encouraging our caregivers to be vaccinated against COVID-19, we are taking an additional step to assure those we serve that we are doing everything possible to keep our hospitals, clinics, and other sites of care safe for them.”
Lourdes and UHS hospitals and emergency rooms remain well prepared to safely care for people with symptoms of a heart attack, stroke, respiratory distress, emergent mental-health concerns, or other serious illness or injury.
Many health care workers and first responders are receiving the earliest wave of available vaccines, as these professions are exposed to COVID-19 at higher rates. Residents of long-term care facilities and those with high-risk health conditions also are slated to receive vaccines early, per guidelines from the CDC.
When COVID-19 vaccines are available for the general public, Lourdes and UHS will share information about the availability of the vaccines through multiple communication channels.